
ABOUT IDRS
IDRS Labs, a specialty pharmaceutical organization, is a leading product development partner to pharmaceutical organizations globally. Incorporated in 2012 by established scientists-turned-entrepreneurs, IDRS is recognized for delivering some of the most complex products – right from inception, development, technology transfer, scale-up, bio-equivalence studies, manufacturing of registration batches, to support for regulatory approval.

INTERNAL ODOUR & PREVALANCE
Ostomies involve "surgery to create an abdominal wall opening (stoma) from an area inside the
body to the outside". This procedure is typically used to treat certain diseases of the digestive
or urinary systems. Ostomies can be permanent, necessitating the removal of an organ, or
temporary, allowing the organ time to heal.
Common types: Ileostomy, Colostomy and Jejunostomy

INTERNAL ODOUR: A VITAL CONCERN
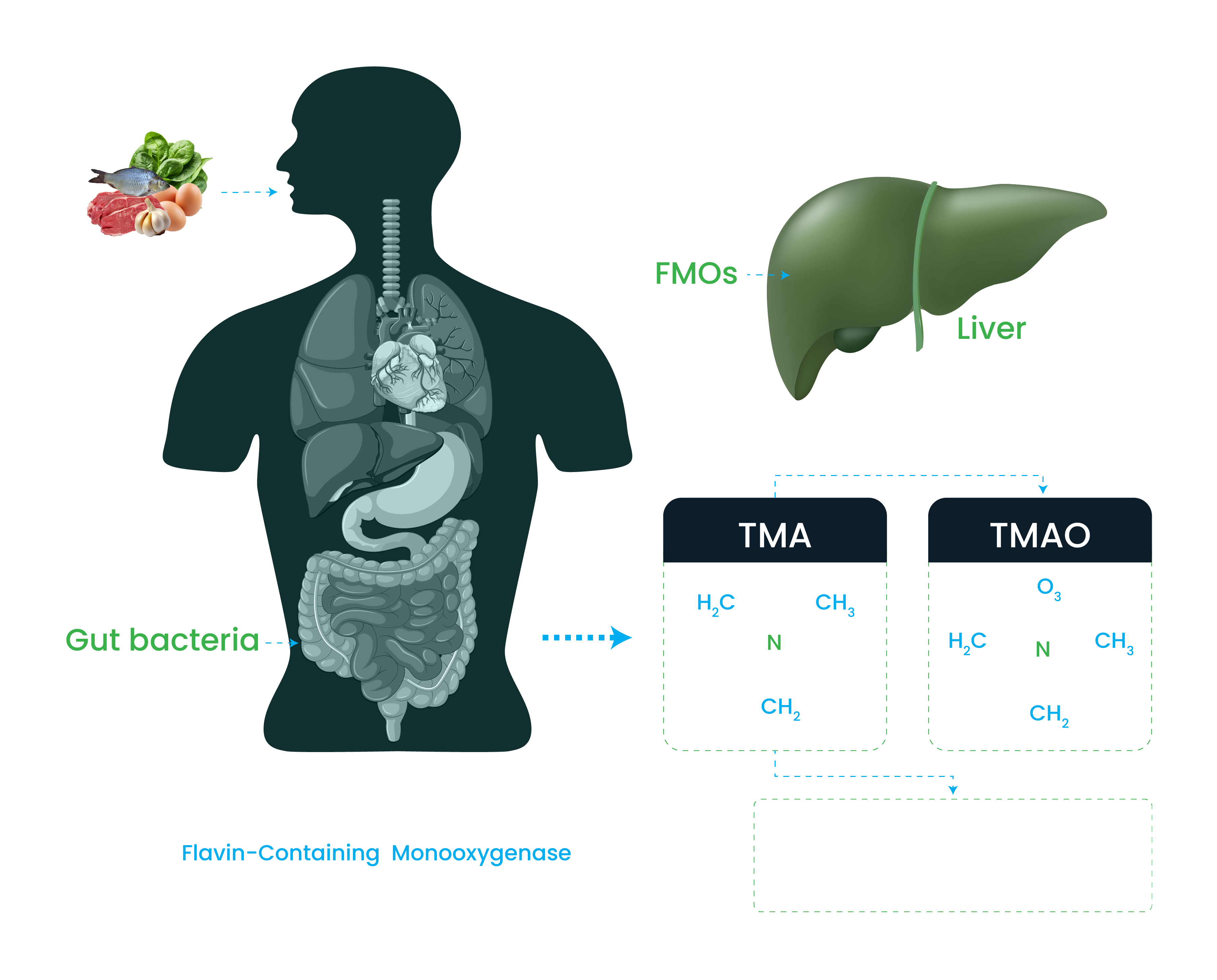
Understanding the pathophysiology of internal odour
Trimethylaminuria is responsible for generation of odour in urine and faces.
- Accumulation of Trimethylamine (TMA) occurs mainly due to reduction in the conversion of Trimethylamine (TMA) to less odorous Trimethylamine-N-oxide (TMAO).
- This conversion is mediated via FMOs enzyme in the liver.
- FMOs enzyme activity is compromised due to mutations in the inFMOs genes.
- Non-functional or structurally altered enzyme impede Trimethylamine (TMA) conversion into Trimethylamine-N-oxide (TMAO), causing accumulation of Trimethylamine (TMA) and subsequent body odour.

MANAGING ODOUR IN OSTOMIES & INCONTINENCE
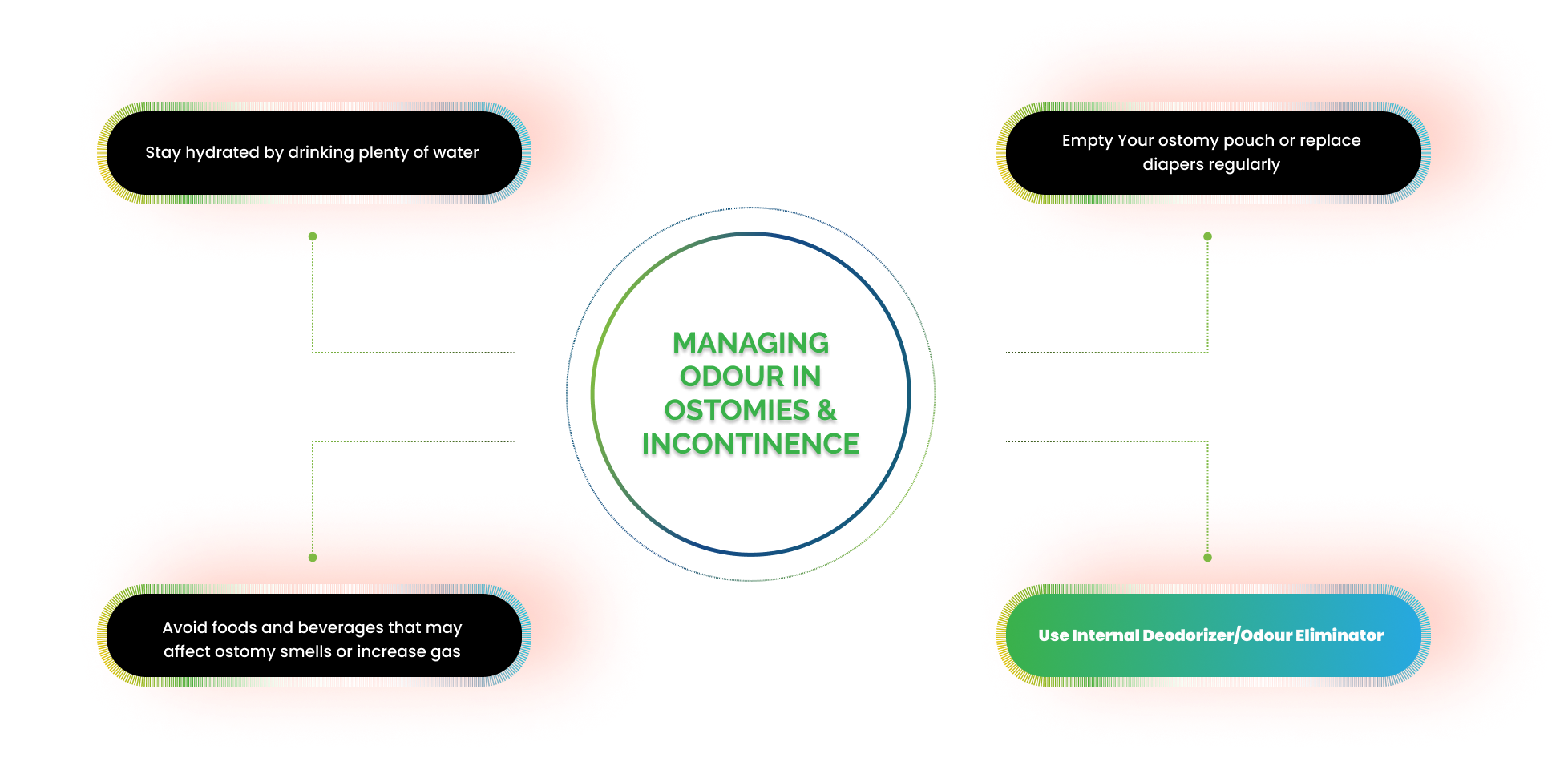
LD developed by IDRS Labs is a strong Internal Deodorant with multiple applications

ABOUT LD
LD contains Sodium Copper Chlorophyllin
(SCC) 100 mg which is a water soluble chlorophyll
derivative extracted from leaves of white mulberry
(Morus Alba L.) of the family Moraceae.
LD is used as a deodorizing agent,
neutralizing odour-causing compounds.
Sodium Copper Chlorophyllin was studied for more
than 70 years for its applications in the multiple
indications including fecal incontinence and as an
effective internal deodorant.
In 1994, USFDA has granted OTC status to SCC. Since
then it is used in the multiple countries including
Japan, Australia, China etc. as an Internal Deodorant.

 Indications
Indications
LD as an Internal Deodorant, helps neutralize body odours in
- Colostomy & Ileostomy
- Urinary & Fecal Incontinence
 Dosage
Dosage
- Take 1 tablet in the morning and 1 tablet in the evening orally with a glass of water, at least one hour before meal.
- Do not exceed 03 Tablets per day. For long term use consult your physician.

WHY TO CHOOSE LD
Research & Publications
for 70+ Years
Rigor in SCC Sourcing
Enhanced Stability
Better Absorption &
Bioavailability
Manufactured as per
GMP standards






ATTRIBUTES OF LD

MECHANISM OF ACTION
- LD enhances the availability of FMOS enzyme which in turn helps in converting Trimethylamine (TMA) to less odorous Trimethylamine-N-oxide (TMAO).
- LD can also directly reduce the amount of Trimethylamine (TMA).
- LD may employ one of the four following mechanisms for reducing Trimethylamine (TMA) and in turn reducing the odour.